Comprehensive Guide to Cosentyx: Benefits, Risks, and Essential Safety Tips
Discover everything about Cosentyx, including its uses, side effects, safety precautions, and how it can effectively treat plaque psoriasis. Learn vital tips for safe administration and monitoring to maximize benefits and minimize risks. A comprehensive guide for patients considering or using Cosentyx.
Comprehensive Guide to Cosentyx: Benefits, Risks, and Essential Safety Tips
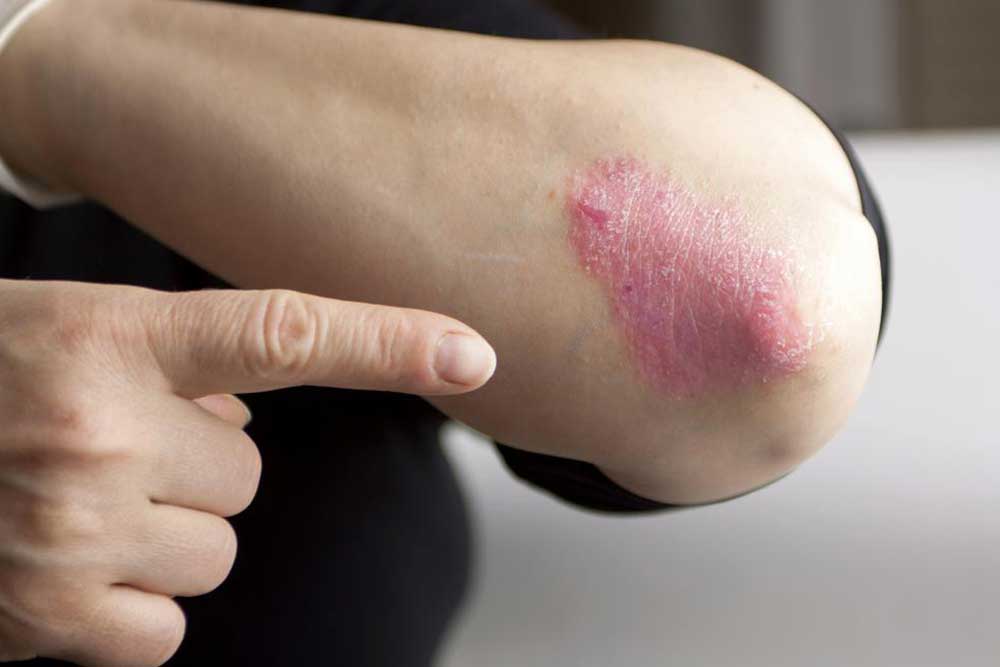
Cosentyx, marketed by Novartis, is a groundbreaking medication widely used in the treatment of plaque psoriasis—a chronic and often debilitating skin disorder that manifests as red, inflamed, and scaly patches on the skin surface. This drug has transformed the management of psoriasis and other related autoimmune conditions, offering relief to millions worldwide. Understanding how Cosentyx works, its potential side effects, and the necessary precautions can help patients and healthcare providers optimize its benefits while minimizing risks.
At the core of Cosentyx's effectiveness is secukinumab, a powerful monoclonal antibody designed to target and inhibit interleukin-17A, a cytokine directly involved in the inflammatory processes underlying psoriasis and other autoimmune diseases. By suppressing this inflammatory mediator, Cosentyx reduces the severity and extent of skin lesions, improves quality of life, and helps control disease outbreaks. However, because it modulates immune responses, proper administration and careful monitoring are critical to avoid adverse effects.
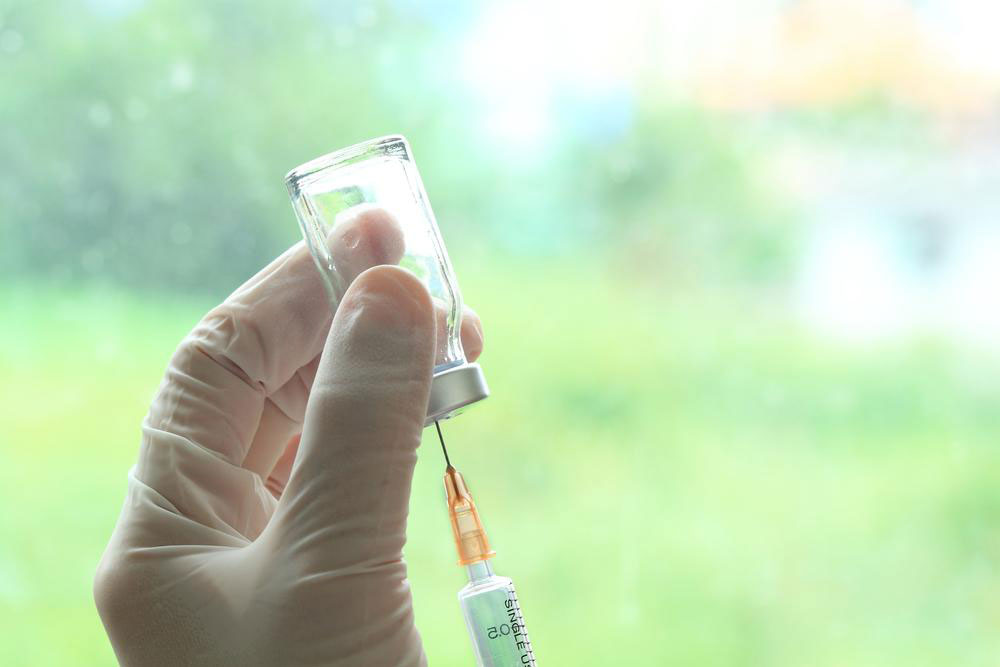
It is crucial for patients to adhere strictly to the dosing schedules prescribed by their healthcare providers. The initial phase typically involves weekly injections over a month to establish control over symptoms, followed by a maintenance phase that involves monthly doses. This regimen helps sustain the therapeutic effects while reducing medication exposure.
Cosentyx comes in a clear to pale yellow solution, designed for subcutaneous injection. Patients should inspect the solution before use—if it appears cloudy, discolored, or contains particulates, it should not be administered. Proper storage and handling are also important to maintain medication potency.
Like all medications, Cosentyx may cause side effects. Common reactions include sore throat, diarrhea, hives, upper respiratory tract infections, and mild skin reactions. Serious adverse effects are less frequent but can occur, such as increased susceptibility to infections, allergic reactions, or rare autoimmune conditions. Patients should be vigilant about symptoms indicating infections like fever, persistent cough, or pus formation and seek medical attention promptly.
Pre-treatment assessment includes screening for active tuberculosis, hepatitis B or C, and other infections, as Cosentyx may exacerbate these conditions. Healthcare providers should also evaluate for history of Crohn’s disease or other inflammatory bowel diseases, as these conditions can sometimes worsen under therapy.
Furthermore, women planning pregnancy or breastfeeding should discuss potential risks and benefits with their healthcare team. Although data on safety during pregnancy is limited, caution is advised, and alternative treatments might be considered.
In conclusion, Cosentyx has emerged as a highly effective solution for managing plaque psoriasis and other autoimmune diseases, significantly improving patients’ lives. However, understanding its mechanism, adhering to prescribed dosing, recognizing side effects, and maintaining regular medical follow-up are essential steps to ensure safe and successful treatment outcomes.