Comprehensive Guide to Biosimilar Medications: Benefits, Uses, and Key Considerations
This comprehensive guide explores biosimilar medications, focusing on their role in treating psoriasis and other autoimmune diseases. It details differences from biologics, identifies suitable patients, reviews approved options, and discusses potential side effects. Biosimilars offer cost-effective, effective treatment alternatives, with ongoing research expanding their potential uses. Supervised by healthcare professionals, these drugs are transforming chronic disease management by providing safer and more accessible options for patients worldwide.

Comprehensive Guide to Biosimilar Medications: Benefits, Uses, and Key Considerations
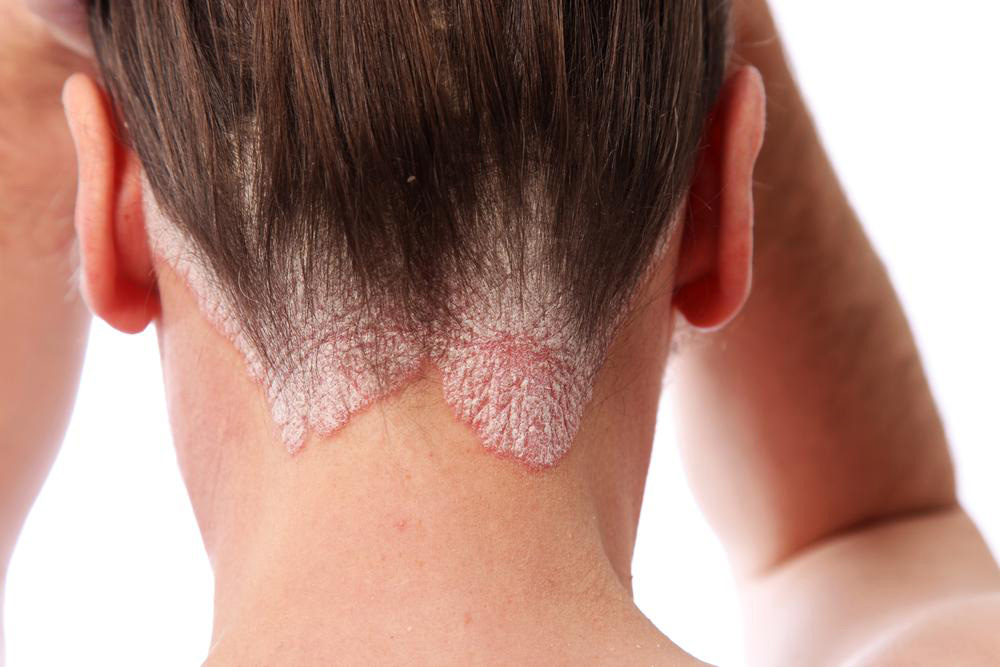
Biosimilar medications have emerged as groundbreaking options in the treatment landscape for various chronic conditions, notably autoimmune diseases like psoriasis. Psoriasis, a prevalent and often distressing skin disorder, manifests in several forms including plaque, inverse, guttate, and erythrodermic psoriasis. It can also impact nails and other parts of the body, significantly affecting quality of life. Advances in biologic and biosimilar therapies have opened new avenues for effective management of this complex condition, offering hope for better symptom control and improved patient outcomes.
Understanding the Differences Between Biologics and Biosimilars
At the core of modern biologic therapies are complex molecules that target specific components of the immune system, such as tumor necrosis factor-alpha (TNF-alpha), interleukins, and other cytokines involved in inflammatory processes. These biologics are engineered proteins derived from living cells, designed to precisely modulate immune responses. Biosimilars, on the other hand, are highly similar versions of these biologics but are produced by different pharmaceutical companies after the original product’s patent expires.
Unlike generic drugs for small-molecule medications, biosimilars cannot be considered exact copies due to the complex manufacturing processes and inherent biological variability. However, they undergo rigorous comparison studies to demonstrate similarity in efficacy, safety, and quality. They usually have comparable dosing regimens and mechanisms of action, making them an attractive, cost-effective alternative to branded biologics. The development and approval of biosimilars are governed by strict regulatory standards to ensure they meet safety and efficacy benchmarks.
Who Are the Ideal Candidates for Biosimilar Treatments?
Biosimilars are primarily recommended for patients suffering from severe psoriasis who require systemic therapy to manage their symptoms effectively. These medications are also considered suitable for individuals with active infections or compromised immune systems, provided their healthcare providers closely monitor their condition. It is important to note that the effects of biosimilars on pregnant women, fetuses, or infants are still being studied. Consequently, healthcare professionals typically prescribe these medications during pregnancy only when the benefits outweigh potential risks, adhering to strict medical guidelines.
FDA-Approved Biosimilars for Psoriasis and Related Conditions
Reflexes and Inflectra: biosimilars similar to the original medication Remicade, used for autoimmune diseases including psoriasis
Erelzi: biosimilar comparable to Etanercept, commonly prescribed for psoriasis and rheumatoid arthritis
Adalimumab-adbm and Adalimumab-atto: biosimilars similar to Adalimumab (Humira), widely used for psoriasis, Crohn’s disease, and other inflammatory conditions
Possible Side Effects and Risks Associated with Biosimilars
While biosimilars provide numerous benefits, they may also cause adverse effects similar to their reference biologics. Common side effects include:
Headaches and migraines
Injection site reactions such as redness, swelling, and discomfort
Respiratory infections like colds and sinusitis
Flu-like symptoms including chills, fever, and fatigue
Sweating or excessive perspiration
Congestion in the chest or nasal passages
Breathing difficulties or shortness of breath
Abdominal pain or gastrointestinal discomfort
Reactions at the site of injection, such as swelling or pain
It’s important to recognize that, although biosimilars are generally safe, they should always be administered under the supervision of qualified healthcare providers. Ongoing research continues to evaluate their long-term safety profile. Reputable medical centers like the Mayo Clinic and UCSF ensure these treatments are provided in controlled environments, emphasizing patient safety and optimal outcomes. As research progresses, biosimilars are expected to play an increasingly vital role in managing not just psoriasis but a broad spectrum of severe autoimmune and inflammatory diseases.