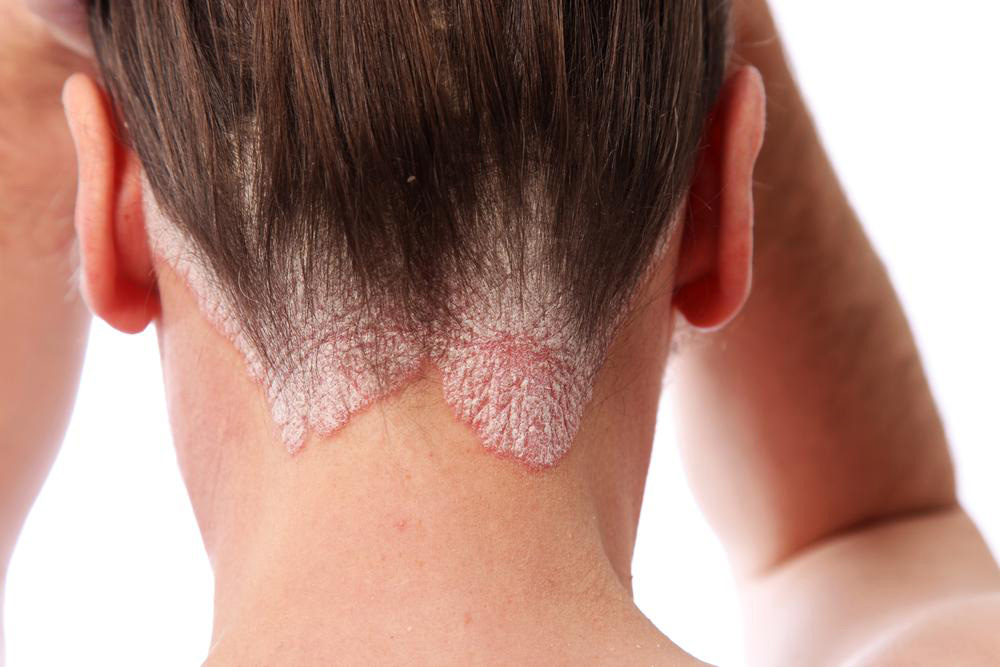
Comprehensive Guide to Cosentyx: Uses, Mechanisms, and Administration
Explore the comprehensive guide to Cosentyx, a targeted biologic used to treat psoriasis, psoriatic arthritis, and ankylosing spondylitis. Learn about its mechanisms, administration, storage, benefits, and safety tips for effective use and improved quality of life.
Comprehensive Guide to Cosentyx: Uses, Mechanisms, and Administration
Cosentyx, known scientifically as Secukinumab, is a cutting-edge prescription medication that has transformed the treatment landscape for autoimmune and inflammatory conditions such as plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis. Approved by the FDA, Cosentyx offers a targeted approach to managing these chronic diseases, providing relief from painful symptoms and improving quality of life for countless patients. This detailed guide explains how Cosentyx works, its benefits, proper administration techniques, storage instructions, and important safety considerations to ensure optimal use.
Understanding the Medical Conditions Treated by Cosentyx
Chronic autoimmune conditions like psoriasis and psoriatic arthritis affect millions worldwide. Psoriasis manifests through skin inflammation, redness, and thick, scaly patches, often leading to discomfort and social stigma. Psoriatic arthritis involves joint pain, swelling, and stiffness, potentially causing permanent joint damage if untreated. Ankylosing spondylitis impacts the spine and the sacroiliac joints, resulting in chronic back pain and mobility issues. These conditions are primarily driven by abnormal immune responses, particularly involving a cytokine known as interleukin-17A (IL17A).
The Science Behind Cosentyx: How It Works
At the core of Cosentyx's effectiveness is its ability to target and neutralize IL17A, a pro-inflammatory cytokine produced predominantly by Th17 cells. In autoimmune diseases, excessive IL17A triggers an inflammatory cascade that leads to the characteristic skin and joint symptoms. Secukinumab, the active ingredient in Cosentyx, is a monoclonal antibody designed to bind selectively to IL17A. By doing so, it blocks this cytokine from binding to its receptor on various cells, thereby preventing downstream inflammatory responses. This targeted action not only reduces inflammation but also helps restore normal immune function, alleviating symptoms and preventing disease progression.
Benefits and Clinical Effectiveness
Patients treated with Cosentyx often report significant improvements in skin clearance, joint pain reduction, and overall quality of life. Clinical trials have demonstrated that a substantial percentage of patients achieve clear or almost clear skin within weeks of starting therapy. In psoriatic arthritis, many patients experience decreased joint swelling and improved mobility. Additionally, long-term studies suggest that Cosentyx maintains its efficacy over time with consistent use. Its targeted mechanism results in fewer systemic side effects compared to traditional immunosuppressants, making it a preferred choice for many healthcare providers.
Administration Techniques and Dosing Schedule
Cosentyx is administered via subcutaneous injection, which requires proper technique for safety and efficacy. Patients or caregivers should be trained on correct injection methods, including site selection, needle handling, and disposal procedures. The typical initial dosing regimen involves weekly injections for the first four weeks to quickly establish therapeutic levels. Once stabilization is achieved, treatment continues with monthly injections to maintain symptom control.
Storage and Handling Guidelines
To preserve medication potency, Cosentyx should be stored in a refrigerator at 2°C to 8°C (36°F to 46°F). It should be kept away from direct sunlight and not frozen. Before administration, the medication may be allowed to reach room temperature, making injection more comfortable. It’s important to inspect the solution before use; it should be clear and light yellow without particles or discoloration. Proper disposal of used needles and cartridges in sharps containers is essential for safety.
Safety Considerations and Side Effects
While Cosentyx is generally well-tolerated, some patients may experience side effects such as injection site reactions, upper respiratory infections, or cold symptoms. Rarely, more serious infections or allergic reactions can occur. Patients with a history of certain infections or immunodeficiencies should consult their healthcare provider before starting therapy. Regular medical follow-up allows for monitoring potential adverse effects and adjusting treatment as needed.
Conclusion
Cosentyx represents a significant advancement in the management of plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis. Its targeted approach to inhibiting IL17A offers effective symptom relief with a favorable safety profile. Proper administration, storage, and adherence to medical guidance are vital in maximizing its benefits. Patients considering Cosentyx should have detailed discussions with their rheumatologist or dermatologist to determine suitability and develop a personalized treatment plan. As research continues, Cosentyx remains at the forefront of biologic therapies, offering hope to those suffering from chronic inflammatory diseases.